Pyrometallurgical Processing
Pyrometallurgical Processing is the dominant method for copper production, accounting for 80% of the world’s copper extracted from copper sulfide concentrates. Its key advantages include high adaptability, low energy consumption, and high efficiency.
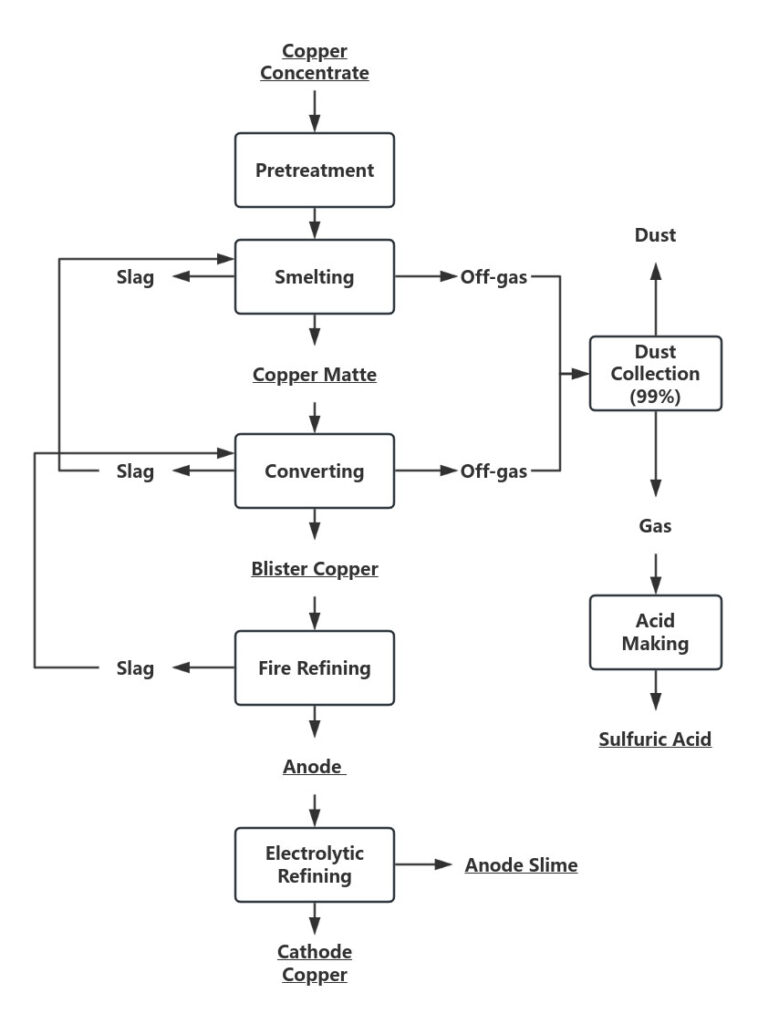
The process involves three main stages:
Smelting – Copper concentrate is melted into matte (a mixture of copper and iron sulfides). Different furnace types are used, including blast furnaces, reverberatory furnaces, electric furnaces, and flash smelting. Regardless of the method, the output is always matte.
Converting – The matte is further processed in a converter to produce blister copper(Approx. 98%).
Refining – Blister copper undergoes fire refining and electrolytic refining to produce high-purity cathode copper. Gold, silver, and other valuable metals are recovered as byproducts.
Capture and fixation of sulfur: The SO₂-rich off-gas from smelting and converting is captured and used to produce sulfuric acid, reducing emissions and adding value.
Hydrometallurgical Processing
Hydrometallurgical processing is a method for extracting copper using a solution-based process. It can be applied to any type of copper ore, be it low or high-grade, oxidized, or sulfide.
The process begins by leaching the ore with a chemical solvent. This dissolves the copper into the solution as ions, while the gangue and other impurities remain undissolved. After leaching, the mixture is clarified and filtered. This separation produces a copper-rich pregnant leach solution (PLS) and a solid waste product called leach residue. As other metals often dissolve alongside the copper, the PLS must be purified. Finally, the purified solution is processed by solvent extraction and electrowinning (SX-EW) to recover the pure copper.
Some economic and process drivers for treating copper ore hydrometallurgically, rather than by smelting, include the following:
1. The relatively high and cyclically variable cost of external smelting and refining charges
2. Limited availability of smelting capacity in some locations
3. High capital cost of installing new smelting capacity
4. The ability to treat high-impurity concentrates, particularly those that contain elements that are deleterious in electrorefining, such as As, Sb and F
5. The ability to treat lower-grade concentrates on site and increasing overall recovery from the ore body
6. The construction of small leach plants at mine sites, rather than shipping concentrate to large distant smelters, thereby eliminating freight and transport costs
7. Cost-effective generation of sulfuric acid at mine sites for use in heap and agitation leaching
8. Overall lower cost production of copper (if credit can be obtained for the acid production)
